Sustained cytopenias designed as Immune Effector Cell-Associated Hematotoxicity (ICAHT) represent one of the most frequent side effect of CAR-T-cell therapy (30 to 90 % patients in recent real life studies) resulting in significant morbidity, mortality and resources consumption. We report single center experience of early reinjection of previously cryopreserved AHSC (HCB) in that setting.
From July 2021 until January 2023, 27 patients received CAR T-cell therapy (25 Axi-Cel, 2 Brexu-Cel) after Fludarabine and Cyclophosphamide lymphodepletion for relapsed/refractory large B-cell lymphomas (24), mantle lymphoma (2), or follicular lymphoma (1). Median age was 62 years (19-75). CAR-HEMATOTOX score was ≥ 2 for 46% of patients.
All patients received Filgrastim from D0. Hematopoietic recovery was longitudinally evaluated until D90 or censoring for relapse and/or subsequent cytotoxic treatment: severe (CTAE grade ≥ 3) neutropenia, anemia or thrombopenia occurred respectively for 88, 44 and 76% of patients until D28, and 60, 24 and 60% until D90. Median duration of severe neutropenia was 7 days (0-39) and 28 days for severe thrombocytopenia (0-118). Prolonged neutropenia occurred in 18/25 evaluable patients, mostly with a biphasic course (intermittent recovery and aplastic phenotype for 14 and 4 patients respectively). CAR-HEMATOTOX score was significantly correlated with sustained thrombopenia (p = 0,005) but only marginally with ICAHT (p = 0,054) and not with severe neutropenia (p = 0,44) . We observed significant correlation of severe ICAHT with progressive disease at CAR T-cell infusion (p = 0,015), platelets nadir < 30 giga/l before D28 (p < 0,005) and CAR T-cell expansion (> 200/µl) at D7 (p = 0,023).
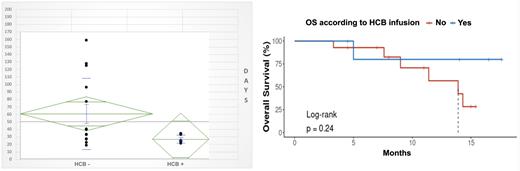
7 patients received HCB (no conditioning) at a median D24 (15-41) post CAR T-cell infusion: after D14 for patients with aplastic phenotype, or for cytopenic recurrence for patients with biphasic course. Filgrastim was pursued or reintroduced until neutrophil recovery (> 1 giga/l) which occurred after a median of 10 days (4-16); platelet recovery (> 20 giga/l and > 50 giga/l) occurred after a median of respectively 12 (8-14) and 17 (11-22) days. If we consider D0 as CAR T-cell infusion, neutrophil recovery occurred at median D37 (22-49) and platelet recovery (> 50 giga/l) at median D37 (26-63). Median complete hematopoietic reconstitution (defined as PMNC > 1 giga/l, and Hb > 100 g/l, and PLT > 100 giga/l) was significantly shorter (median 23 days, 14-30) for patients receiving HCB compared with patients with prolonged cytopenia (> 28 days) who do not receive HCB (60 days, p = 0,018). Transfusion and hematopoietic growth factors (HGF) consumption was significantly reduced after HCB but we observe no difference between 2 groups considering hospitalization, EFS or OS. 2 patients receiving HCB experienced late recurrence of cytopenia (after D90) requiring intermittent HGF support but no transfusion or hospitalization.
Discussion - Perspectives
Recent case series highlighted clinical feasibility and safety and of this approach across a broad population of pediatric and adult patients: we validate this strategy in a more homogeneous and systematic practice. As study population is small, we failed to demonstrate that earlier application of an HCB is associated with superior survival outcomes as shown by others. We agree with recent updated recommendation of experts panel considering application of HCB without prior conditioning chemotherapy for grade ≥ 3 ICAHT beyond D14 (so considered early) if AHSC graft is readily available. Prophylactic AHSC harvest in high-risk candidates has been proposed but collection process may add to already high logistic burden and costs of CAR T-cell therapy in the lymphoma setting. Conversely, cryopreserved ASHC is mainly available for patients with myeloma and this approach would be readily proposed and useful for around 20% patients as recently described.
Gagelmann N et al. Hematopoietic stem cell boost for persistent neutropenia after CAR T-cell therapy: a GLA/DRST study. Blood Adv. 2023;7(4):555
Rejeski K et al, Immune Effector Cell-Associated Hematotoxicity (ICAHT): EHA/EBMT Consensus Grading and Best Practice Recommendations. Blood. 2023 doi: 10.1182/blood.2023020578
Davis JA et al. Efficacy and safety of CD34+ Stem Cell Boost for Delayed hematopoietic Recovery After BCMA Directed CAR T-cell Therapy. Transplant Cell Ther. 2023 doi: 10.1016/j.jtct.2023.05.012
Disclosures
No relevant conflicts of interest to declare.